MIT chemical engineers have devised an efficient way to convert carbon dioxide to carbon monoxide, a chemical precursor that can be used to generate useful compounds such as ethanol and other fuels.
If scaled up for industrial use, this process could help to remove carbon dioxide from power plants and other sources, reducing the amount of greenhouse gases that are released into the atmosphere.
“This would allow you to take carbon dioxide from emissions or dissolved in the ocean, and convert it into profitable chemicals. It’s really a path forward for decarbonization because we can take CO2, which is a greenhouse gas, and turn it into things that are useful for chemical manufacture,” says Ariel Furst, the Paul M. Cook Career Development Assistant Professor of Chemical Engineering and the senior author of the study.
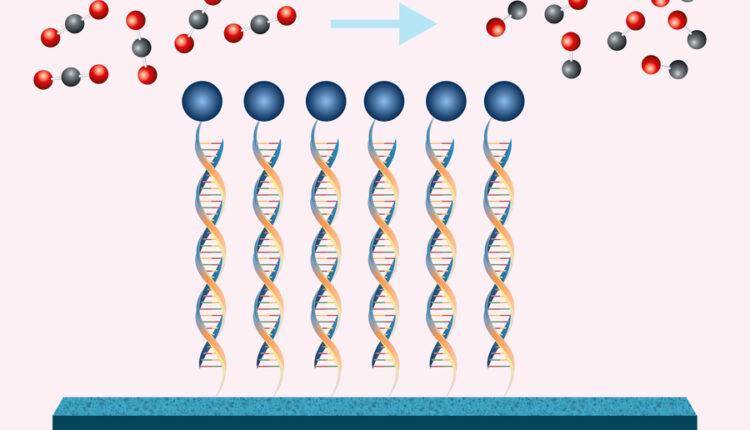
The new approach uses electricity to perform the chemical conversion, with help from a catalyst that is tethered to the electrode surface by strands of DNA. This DNA acts like Velcro to keep all the reaction components in close proximity, making the reaction much more efficient than if all the components were floating in solution.
Furst has started a company called Helix Carbon to further develop the technology. Former MIT postdoc Gang Fan is the lead author of the paper, which appears in the Journal of the American Chemical Society Au. Other authors include Nathan Corbin PhD ’21, Minju Chung PhD ’23, former MIT postdocs Thomas Gill and Amruta Karbelkar, and Evan Moore ’23.
Breaking down CO2
Converting carbon dioxide into useful products requires first turning it into carbon monoxide. One way to do this is with electricity, but the amount of energy required for that type of electrocatalysis is prohibitively expensive.
To try to bring down those costs, researchers have tried using electrocatalysts, which can speed up the reaction and reduce the amount of energy that needs to be added to the system. One type of catalyst used for this reaction is a class of molecules known as porphyrins, which contain metals such as iron or cobalt and are similar in structure to the heme molecules that carry oxygen in blood.
During this type of electrochemical reaction, carbon dioxide is dissolved in water within an electrochemical device, which contains an electrode that drives the reaction. The catalysts are also suspended in the solution. However, this setup isn’t very efficient because the carbon dioxide and the catalysts need to encounter each other at the electrode surface, which doesn’t happen very often.
To make the reaction occur more frequently, which would boost the efficiency of the electrochemical conversion, Furst began working on ways to attach the catalysts to the surface of the electrode. DNA seemed to be the ideal choice for this application.
“DNA is relatively inexpensive, you can modify it chemically, and you can control the interaction between two strands by changing the sequences,” she says. “It’s like a sequence-specific Velcro that has very strong but reversible interactions that you can control.”
To attach single strands of DNA to a carbon electrode, the researchers used two “chemical handles,” one on the DNA and one on the electrode. These handles can be snapped together, forming a permanent bond. A complementary DNA sequence is then attached to the porphyrin catalyst, so that when the catalyst is added to the solution, it will bind reversibly to the DNA that’s already attached to the electrode — just like Velcro.
Once this system is set up, the researchers apply a potential (or bias) to the electrode, and the catalyst uses this energy to convert carbon dioxide in the solution into carbon monoxide. The reaction also generates a small amount of hydrogen gas, from the water. After the catalysts wear out, they can be released from the surface by heating the system to break the reversible bonds between the two DNA strands, and replaced with new ones.
An efficient reaction
Using this approach, the researchers were able to boost the Faradaic efficiency of the reaction to 100 percent, meaning that all of the electrical energy that goes into the system goes directly into the chemical reactions, with no energy wasted. When the catalysts are not tethered by DNA, the Faradaic efficiency is only about 40 percent.
This technology could be scaled up for industrial use fairly easily, Furst says, because the carbon electrodes the researchers used are much less expensive than conventional metal electrodes. The catalysts are also inexpensive, as they don’t contain any precious metals, and only a small concentration of the catalyst is needed on the electrode surface.
By swapping in different catalysts, the researchers plan to try making other products such as methanol and ethanol using this approach. Helix Carbon, the company started by Furst, is also working on further developing the technology for potential commercial use.
The research was funded by the U.S. Army Research Office, the CIFAR Azrieli Global Scholars Program, the MIT Energy Initiative, and the MIT Deshpande Center.

Comments are closed.