HKUMed discovers a new metabolic route for liver cancer paving the way for new therapeutic opportunities
Researchers from the Department of Pathology, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) have identified a new metabolic mechanism in liver cancer cells, by which macropinocytosis, an endocytic process, is used to engulf extracellular proteins as fuels to support the unlimited growth of hepatocellular carcinoma (HCC) in hypoxia (oxygen deprivation). This discovery indicates that targeting macropinocytosis might represent a novel and effective therapeutic approach for HCC. The findings have now been published in Nature Communications [link to the publication].
Background
HCC is the most common form of primary liver cancer. In Hong Kong, HCC is the fifth most common and the third most lethal cancer. Despite the increasing therapeutic options, the median life expectancy of HCC patients in advanced stage is still less than two years.
Blood delivers oxygen, an important nutrient for HCC. However, the oxygen content in solid tumours including HCC is usually lower than normal organs due to the abnormally high growth speed of tumours, far exceeding the growth of blood vessels. HCC cells are resilient to oxygen deprivation as they have high adaptability to this ‘nutrient stress’ through stabilising a transcription factor called hypoxia-inducible factor-1 (HIF-1). The HKUMed study has identified a novel gene, EHD2, regulated by HIF-1, which instigates a metabolic programme dependent on macropinocytosis to facilitate HCC cells to adapt and proliferate under ‘nutrient stress’. The study also showed that therapeutically targeting the macropinocytosis pathway could suppress HCC effectively.
Research methods and findings
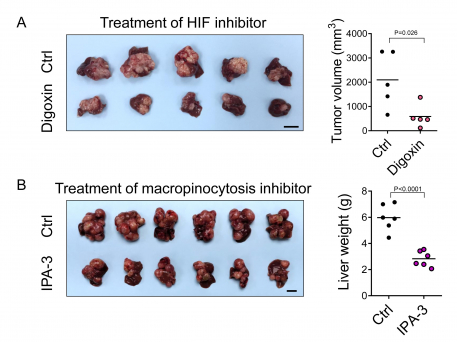
Macropinocytosis is an endocytic pathway that allows cells to engulf extracellular proteins. Through confocal microscopy, it was shown that hypoxia could cause HCC cells to exhibit 5-10 folds of induction of macropinocytic level. Through mass spectrometry analysis, the HKUMed team also found that the engulfed proteins were digested and degraded into amino acids which in turn served as fuel to support HCC cell proliferation. This process was mediated by HIF-1 which transcriptionally activated a novel gene called EH domain-containing protein 2 (EHD2), driving membrane ruffle formation, is the first and critical step in macropinocytosis. Deletion of EHD2 in mice prevented the development of HCC. The treatment of a HIF inhibitor, digoxin, or a macropinocytosis inhibitor – IPA-3, also achieved a more than 50% inhibition of the tumour burden in mouse HCC models (Figure A, Figure B). More importantly, EHD2 was significantly over-expressed by at least two folds in about 40% of HCC patients. Taken together, the study demonstrated that HIF-1/EHD2-mediated macropinocytosis supports HCC growth. The inhibition of macropinocytosis represents a potent therapeutic approach for HCC.
Research significance
‘Liver cancer has poor prognosis, partly because its cancer cells are smart enough to find ways to survive in deprived conditions. Understanding the biology and metabolism of liver cancer is thus fundamental to the identification of effective therapeutic targets and treatments. In this study, we found that liver cancer cells would start to adapt alternative and unusual metabolic programme to scavenge proteins from the environment as nutrient source. Also, the HIF-1/EHD2 pathway identified supports macropinocytosis-mediated metabolic reprogramming in cancer, providing an important molecular basis for the design of innovative cancer therapeutic strategies against macropinocytosis. It is important to note that hypoxia is not limited to liver cancer but all solid cancers. Therefore, our finding will be applicable to other cancer types as well,’ said Dr Carmen Wong Chak-lui, Associate Professor, Department of Pathology, School of Clinical Medicine, HKUMed.
About the research team
The research was led by Dr Carmen Wong Chak-lui, Associate Professor, Department of Pathology, School of Clinical Medicine, HKUMed, Principal Investigator, State Key Laboratory of Liver Research (The University of Hong Kong). Dr Misty Zhang Shuo, Post-doctoral Fellow, Department of Pathology, School of Clinical Medicine, HKUMed, was the first author, with the help of Dr Jane Cui Di, Research Assistant; Dr Derek Lee, Dr David Chiu Kung-chun and Dr Goh Chi Ching, Post-doctoral Fellows. Other members of the research team include Mr Vincent Yuen Wai-hin, Miss Jacinth Cheu Wing-sum, Mr Macus Bao Haoran, Miss Bowie Wong Po-yee and Miss Carrie Chen Yiling, postgraduate students; Miss Aki Tse Pui-wah, Technical Officer, Department of Pathology, School of Clinical Medicine, HKUMed; Dr Wong Chun-ming, Associate Professor, Department of Pathology, School of Clinical Medicine, HKUMed; Principal Investigator, State Key Laboratory of Liver Research (The University of Hong Kong); Professor Irene Ng Oi-lin, Loke Yew Professor in Pathology, Chair Professor of Pathology, Department of Pathology, School of Clinical Medicine, HKUMed; Director, State Key Laboratory of Liver Research (The University of Hong Kong).
Acknowledgements
This study was supported by Research Grant Council General Research Fund (17115218) and National Natural Science Foundation of China (NSFC) Excellent Young Scientists Fund (Hong Kong and Macau) (82022077).

Comments are closed.